Question 1:
Choose the correct answer.
A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is
suddenly increased.
(a) What is the initial effect of the change on vapour pressure ?
(b) How do rates of evaporation and condensation change initially ?
(c) What happens when equilibrium is restored finally and what will be the final vapour pressure ?
Answer:
(a) Vapour pressure decreases since equilibrium is disturbed and the rate of condensation falls considerably.
(b) Rate of evaporation increases while that of condensation falls.
(c) On restoration of the equilibrium again the vapour pressure becomes the same.
Question 2:

Answer:

Question 3:

Answer:

Question 4:

Answer:

Question 5:

Answer:

Question 6:

Answer:

Question 7:
Explain why pure liquids and solids can be ignored while writing the equilibrium constant expression ?
Answer:
While writing expressions for equilibrium constant involving gases, liquids and solids, only the concentrations of the gases are taken into consideration, since the change in concentration of pure liquids and solids is negligibly small as compared to that of gases and is taken as constant.
Question 8:

Answer:

Question 9:

Answer:

Question 10:
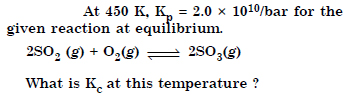
Answer:

Question 11:

Answer:

Question 12:

Answer:

Question 13:

Answer:

Question 14:

Answer:

Question 15:

Answer:

Question 16:

Answer:

Question 17:

Answer:

Question 18:

Answer:

Question 19:

Answer:

Question 20:

Answer:

Question 21:

Answer:

Question 22:

Answer:

Question 23:

Answer:

Question 24:

Answer:

Question 25:
Does the number of moles of reactions products increase, decrease or remain same when each of the following equilibria is subjected to a decrease in pressure by
increasing the volume ?

Answer:
(a) Increases
(b) decreases
(c) remaining same (By applying Le Chatelier’s Principle.)
Question 26:

Answer:

Question 27:

Answer:

Question 28:

Answer:

Question 29:

Answer:

Question 30:

Answer:

Question 31:

Answer:

Question 32:

Answer:

Question 33:

Answer:

Question 34:

Answer:

Question 35:

Answer:

Question 35:

Answer:

Question 36:
What will be the conjugate bases for the Bronsted acids : HF, H2SO4 and HCO3 ?
Answer:

Question 37:
Write the conjugate acids for the following bases : NH2, NH3 and HCOO.
Answer:

Question 38:

Answer:

Question 39:
Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base : (a) OH– (b) F– (c) H+ (d) BCl3.
Answer:

Question 40:
Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base :
(a) OH–
(b) F–
(c) H+
(d) BCl3.
Answer:

Question 41:
The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it.
Answer:

Question 42:

Answer:

Question 43:
The ionization constant of phenol is 1.0 × 10–10. What is the concentration of phenate ion in 0.05 M solution of phenol ? What will be its degree of ionization if the solution is also 0.01 M in sodium phenate ?
Answer:

Question 44:

Answer:

Question 45:
The ionization constant of acetic acid is 1.74 × 10–4. Calculate the degree of dissociation of acetic acid in its 0.05 M solution. Calculate the concentration of acetate ion in the solution and its pH.
Answer:

Question 46:
It has been found that the pH of a 0.01 M solution of an organic acid is 4.15. Calculate the concentration of the anion, the ionization constant of the acid and its pKa.
Answer:

Question 46:
Assuming complete dissociation, calculate the pH of the following solutions :
(a) 0.003 M HCl (b) 0.005 M NaOH
(c) 0.002 M HBr (d) 0.002 M KOH.
Answer:

Question 47:
Assuming complete dissociation, calculate the pH of the following solutions :
(a) 0.003 M HCl (b) 0.005 M NaOH
(c) 0.002 M HBr (d) 0.002 M KOH.
Answer:

Question 48:
Calculate the pH of the following solutions :
(a) 2 g of TlOH dissolved in water to give 2 litres of solution.
(b) 0.3 g of Ca(OH)2 dissolved in water to give 500 ml of solution.
(c) 0.3 g of NaOH dissolved in water to give 200 ml of solution.
(d) 1 ml of 13.6 M HCl is diluted with water to give 1 litre of solution.
Answer:

Question 49:
The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and the pKa of bromoacetic acid.
Answer:

Question 50:

Answer:

Question 52:
What is the pH of 0.001 M aniline solution ? The ionization constant of aniline can be taken as 4.27 × 10–10. Calculate the degree of ionization of aniline in the solution. Also calculate the ionization constant of the conjugate acid of aniline.
Answer:

Question 53:
Calculate the degree of ionization of 0.05 M acetic acid if its pKa value is 4.74. How is the degree of dissociation affected when its solution also contains (a) 0.01 M (b) 0.1 M in HCl ?
Answer:

Question 54:
The ionization constant of dimethylamine is 5.4 × 10–4. Calculate its degree of ionization in its 0.02 M solution. What percentage of dimethylamine is ionized if the solution is also 0.1 M in NaOH ?
Answer:

Question 55:
Calculate the hydrogen ion concentration in the following biological fluids whose pH are given below :
(a) Human muscle fluid 6.83
(b) Human stomach fluid 1.22
(c) Human blood 7.38
(d) Human saliva 6.4.
Answer:

Question 56:
The pH of milk, black coffee, tomato juice, lemon juice and egg white are 6.8, 5.0, 4.2, 2.2 and 7.8 respectively. Calculate corresponding hydrogen ion concentration in each.
Answer:

Question 57:
If 0.56 g KOH is dissolved in water to give 200 ml of solution at 298 K. Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH ?
Answer:

Question 58:
The solubility of Sr(OH)2 at 298 K is 19.23 g/L of solutions. Calculate the concentrations of strontium and hydroxyl ions and the pH of the solution.
Answer:

Question 59:
The ionization constant of propionic acid is 1.32 × 10–5. Calculate the degree of ionization of the acid in its 0.05 M solution and also its pH. What will be its degree of ionization if the solution is 0.01 M in HCl also ?
Answer:

Question 60:
The pH of 0.1 M solution of cyanic acid (HCNO) is 2.34. Calculate the ionization constant of the acid and its degree of ionization in the solution.
Answer:

Question 61:
The ionization constant of nitrous acid is 4.5 × 10–4. Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.
Answer:

Question 62:
A 0.02 M solution of pyridinium hydrochloride has pH = 3.44. Calculate the ionization constant of pyridine.
Answer:

Question 63:
Predict if the solutions of the following salts are neutral, acidic or basic : NaCl, KBr, NaCN, NH4NO3 and KF.
Answer:

Question 64:
The ionization constant of chloroacetic is 1.35 × 10–3. What will be pH of 0.1 M acid and its 0.1 M sodium salt solution ?
Answer:

Question 65:
Ionic product of water at 310 K is 2.7 × 10–14. What is the pH of neutral water at this temperature ?
Answer:

Question 66:

Answer:

Question 67:

Answer:


Question 68:

Answer:

Question 69:
Equal volumes of 0.002 M solutions of sodium iodate and copper chlorate are mixed together. Will it lead to precipitation of copper iodate ? For copper iodate Ksp= 7.4 × 10–8.
Answer:

Question 70:

Answer:

Question 71:
What is the maximum concentration of equimolar solutions of ferrous sulphate and sodium sulphide so that when mixed in equal volumes, there is no precipitation of iron sulphide. For iron sulphide Ksp = 6.3 × 10–18.
Answer:

Question 72:
What is the minimum volume of water required to dissolve 1 g of calcium sulphate at 298 K ? (For calcium sulphate Ksp is 9.1 × 106)
Answer:

Question 73:

Answer:

