Get a better understanding of the concepts of the “Biomolecules” chapter with free NCERT Solutions on Aasoka. Written in an easy-to-understand language, these solutions are designed keeping the latest CBSE syllabus in mind. NCERT Solutions for Class 11 are helpful in preparing for competitive exams including NEET and JEE. Be thorough with all the topics of the chapter and brush up your skills to give excellent performance in the Class 11 exams.
Biology Class 11 “Biomolecules” chapter discusses a variety of living organisms present in our biosphere, the concept of metabolism, enzymes, chemical constituents of living cells, biomacromolecules, how to analyze the chemical composition, polysaccharides, proteins, carbohydrates, lipids, dynamic state of body constituents, etc.
Question 1:
What are macromolecules? Give examples.
Answer:
Macromolecules are complex, large molecules assembled by the polymerisation of simple monomers. They have molecular weights in the range of ten thousand daltons and above. Examples include carbohydrates, proteins and nucleic acids.
Question 2:
What is meant by tertiary structure of proteins?
Answer:
Tertiary Structure of proteins. When secondary proteins undergo a third degree of twisting or torsion formed by additional bonds of both functional groups ; the protein is then called to be in its tertiary structure. The polypeptide chain is twisted and folded to form a stable structure.
Tertiary structure of protein
It is maintained by three types of bonds i.e. ionic bonds, hydrogen bonds and disulphide bond e.g. Myoglobin protein shows a tertiary structure.
Question 3:
(a) Find and write down structure of 10 interesting small molecular weight biomolecules. (b) Find if there is any industry which manufactures the compounds by isolation Find out who are buyers.
Answer:
(a) Small molecular weight biomolecules.
(b) Sugar industry. Concerned with manufacture of sugar. Every man is buying sugar.
Question 4:
Proteins have primary structure. If you are given a method to know which amino acid is at either of two terminal ends of a protein, can you connect this information to purity or homogeneity of a protein ?
Answer:
The terminal ends are always with
—COOH (carboxylic) and —NH2 (amino group). The two terminals are called C—Terminal and N—terminal. This will not help in finding the homogeneity of a protein.
Question 5:
Find out and make a list of proteins used as therapeutic agents. Find other applications of proteins (e.g. Cosmetics etc.)
Answer:
- Antibodies used as vaccines to carry out immunisation of animals.
- Humulin artificially synthesised protein to act as a substitute for insulin.
- Calcitonin
- Fibrinogen
- Antifungal peptides
- Albumins
- Prothrombin
- Proteins which are used as cosmetics :
(a) Casein. (b) Keratin. (c) Albumin.
Question 6:
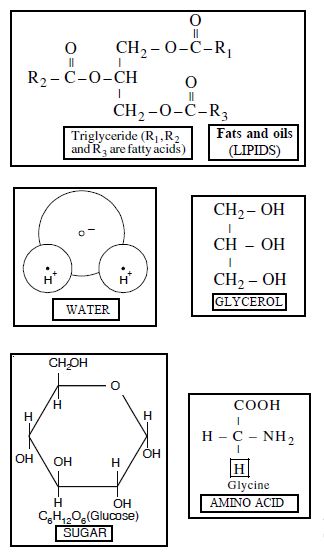
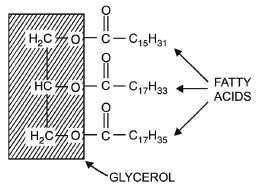
Explain the composition of a triglyceride.
Answer:
Triglycerides have a three –OH group containing glycerol linked to three fatty acid molecules by ester bond. During esterification three molecules of water are eliminated e.g. Tripalmitin, tristearin and triolein (Fig. 9.4).
A triglyceride fat
Question 7:
Can you describe what happens when milk is converted into curds or yoghurt from your understanding of proteins ?
Answer:
When milk is converted into curd or yoghurt, the milk protein (casein) undergoes denaturation i.e. there is a disruption in the native state of the protein. Since this conversion process is carried out by bacteria (Lactobacillus), it is called biological denaturation. Proteins of lactobacillus are used as enzyme. The enzyme utilize lactose sugar present in milk and produce lactic acid that is responsible for the curdling of milk.
Question 8:
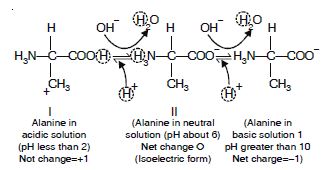
Attempt titrating an amino acid against a weak base and discover the number of dissociating (ionizable) functional groups in the amino acid.
Answer:
Zwitter ion form of amino acid
At a low (acidic) pH, both groups carboxylic and amino group are protonated. As the pH of the solute is raised, the –COOH group of form I can dissociate by donating a proton to the medium. The release of proton results in the formation of carboxylate group –COO–. This structure is shown as form II which is dipolar form of molecule. This form is also called Zwitter ion form and is the isoelectric form of alanine. Thus it has overall charge of zero.
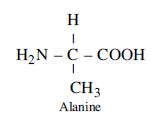
Question 9:
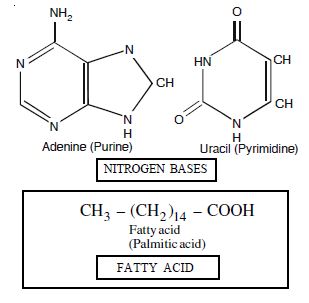
Draw the structure of amino acid Alanine.
Answer:
Structure of amino acid alanine
Question 10:
What are gums made of ? Is fevicol different ?
Answer:
Gums are made of hexoses and pentoses. Gum is formed naturally in plants by the decomposition of cellulose. It is colloidal in nature, soluble in water and insoluble in organic solvents like alcohol. It is closely allied to pectin and contains polysaccharides especially xylose.
Fevicol is a synthetic gum as it is a petrochemical polymer artificially synthesised in industries.
Question 11:
Find out a qualitative test for proteins, fats and oils, amino acids and test any fruit juice, saliva, sweat and wine for them.
Answer:
Test for Proteins
Biuret test. Take 3ml of egg albumin in a test tube and add an equal volume of 10% NaOH. Mix thoroughly and add 0.5% CuSO4 dropwise. Mix well. A purple-violet or pinkish violet colour develops.
Test for Fats
Solubility test. In five test tubes, take 3ml of water, alcohol, ether, benzene and chloroform. To each test tube add 3 drops of lipid. Shake well and note the solubility. The lipid floats on water but it is miscible with ether, benzene and chloroform, while it sinks to the bottom in alcohol.
Question 12:
Find out how much cellulose is made by all the plants in the biosphere and compare it with how much of paper is manufactured by man and hence what is the consumption of plant material by man annually. What a loss of vegetation !
Answer:
The amount of paper manufactured by man exceeds the quantity of cellulose made by the plants in biosphere upto a great extent. The demand for paper is increasing day by day leading to intense deforestation by mankind. This leads to loss of vegetation, the net result of which is a disturbance in ecological equilibrium and loss of plant and animal species.
Question 13:
Describe the important properties of enzymes.
Answer:
The important properties of enzymes are listed below :
- Chemical Nature. The enzymes are proteinic in nature except ribozyme. Some enzymes have additional non-protein (inorganic or organic) substances associated with them for their action.
- Molecular Weight. The enzymes have very high molecular weights. These vary from 6000 for bacterial ferredoxin to 4,600,000 for pyruvate dehydrogenase complex.
- Chemical Activity. The enzymes accelerate a chemical reaction by lowering activation energy.
- Changeless Form. The enzymes combine temporarily with the substrate molecules but are not consumed or changed permanently in the reaction they catalyse. They are produced in same form at the end of reaction.
- Reversibility of Reaction. The enzyme-controlled reactions are reversible.
- High Efficiency. Most enzymes have high turnover number. A molecule of the enzyme catalase from cattle liver decomposes 5,000,000 molecules of hydrogen peroxide to water and hydrogen in one minute at 0C. The turnover number of catalase is thus, 5,000,000 at 0C. The turnover number for carbonic anhydrase present in the RBCs is 36 million. The higher the turnover number, the more efficient is enzyme.
- Action Specificity. The enzymes are specific in action.
- Temperature Sensitivity. The enzymes function best at an optimum temperature. The optimum temperature for human enzymes is 35 to 40C—close to human body temperature. The enzyme activity decreases with decrease as well as increase in temperature, and stops at 0C and above 80C. The enzymes of bacteria inhabiting hot springs have an optimum temperature of 70C or more.
- pH Sensitivity. The enzymes show maximum activity at an optimum pH (6 – 8) but enzymes have specific pH for their action.
- An extremely small quantity of enzyme is required to bring about a measurable change in the rate of reaction.