Question 1:
Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different ? Justify your answer.
Answer:
Two isotopes of the same element have the same first ionization enthalpies because of the same effective nuclear charges.
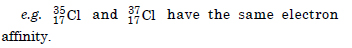
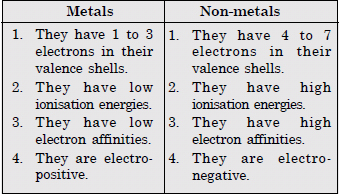
What are the major differences between metals and non-metals ?
Answer:
Question 3:
Use the periodic table to answer the following questions :
(a) Identify an element with five electrons in the outer subshell.
(b) Identify an element that would tend to lose two electrons.
(c) Identify an element that would tend to gain two electrons.
(d) Identify the group having metal, nonmetal, liquid as well as gas at the room temperature.
Answer:
(a) Fluorine (b) Magnesium
(c) Oxygen
(d) Group 1.
Question 4:
The increasing order of reactivity among group 1 elements is Li < Na < K < Rb < Cs whereas that among group 17 elements is F > Cl > Br > I. Explain.
Answer:
This is because in the group 1 ionisation energy decreases on moving from top to bottom and in group 17, standard reduction potential goes on decreasing on moving from top to bottom.
Question 5:
Write the general outer electronic configuration of s–, p–, d– and f– block elements.
Answer:
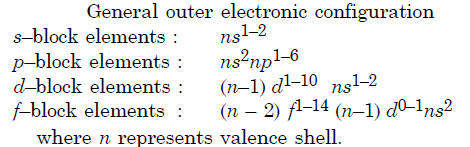
Question 6:
Find energy of each of photons which
(i) correspond to light of frequency 3 × 105 Hz
(ii) have wavelength of 0.50 Å.
Answer:
Question 7:

Answer:
Frequency of a light wave,

Question 8:

(a) the least reactive element.
(b) the most reactive metal.
(c) the most reactive non-metal.
(d) the least reactive non-metal.
e) the metal which can form a stable
binary halide of the formula MX2 (X = halogen).
(f) the metal which can form a
predominantly stable covalent halide of the
formula MX (X = halogen) ?
Answer:
(a) (V),
(b) (II),
(c) (III),
(d)(VI),
(e) (I),
(f) (II).
Question 9:
Predict the formulae of the stable binary compounds that would be formed by the combination of the following pairs of elements :
(a) Lithium and oxygen
(b) Magnesium and nitrogen
(c) Aluminium and iodine
(d) Silicon and oxygen.
(e) Phosphorus and Fluorine
(f) Element 71 and fluorine.
Answer:

Question 10:
In the modern periodic table, the period indicates the value of :
Answer:
principal quantum number
Question 11:
Which of the following statements related to the modern periodic table is incorrect ?
Answer:
The d-block has 8 columns, because a maximum of 8 electrons can occupy all the orbitals in a d-subshell.
Question 12:
Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell ?
Answer:
Nuclear mass
Question 13:
The size of isoelectronic species— F–, Ne and Na+ is affected by :
Answer:
nuclear charge (Z)
Question 14:
Which one of the following statements is incorrect in relation to ionization enthalpy ?
Answer:
Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value.
Question 15:
Considering the elements B, Al, Mg and K, the correct order of their metallic character is :
Answer:
K > Mg > Al > B.
Question 16:
Considering the elements B, C, N, F and Si, the correct order of their non-metallic character is :
Answer:
F > N > C > Si > B.
Question 17:
Considering the elements F, Cl, O and N, the correct order of their chemical reactivity in terms of oxidizing property is :
Answer:
F > Cl > O > N
