Question 1:
Define environmental chemistry.
Answer:
Refer to Basics and Basis.
Question 2:
Explain tropospheric pollution in 100 words.
Answer:
It extends upto the height of about 10 km from sea level. The tropospheric pollution occurs due to the presence of undesirable solid or gaseous particles in the air. The following are the major gaseous and particulate pollutants present in the atmosphere.
1. Gaseous air pollutants. These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants. They cause asthma, bronchitis and can lead to acute respiratory diseases.
2. Particulate air pollutants. These are dust, moist, fumes, smoke, smog etc.
Question 3:
Carbon monoxide gas is more dangerous than carbon dioxide, why ?
Answer:
Carbon monoxide (CO) when inhaled reacts with haemoglobin (Hb) to form complex carboxy haemoglobin (COHb). Therefore, haemoglobin can't carry oxygen to the various parts of the body. On the other hand, the presence of carbon dioxide can lead only to greenhouse effect causing global warming.
Question 4:
Which gases are responsible for greenhouse effect ? Name them.
Answer:
The greenhouse effect is caused by the following gases which are capable of trapping heat energy.
(i) Carbon dioxide
(ii) methane
(iii) ozone
(iv) chlorofluorocarbon compounds (CFC's)
(v) water vapours.
Question 5:
Statues and monuments in India are affected by acid rain. How ?
Answer:
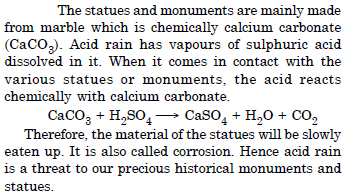
Question 6:
What are smogs ? How are classical and photochemical smog different ?
Answer:
The smog is a combination of smoke and fog. The difference between classical and photochemical smogs are as follows :
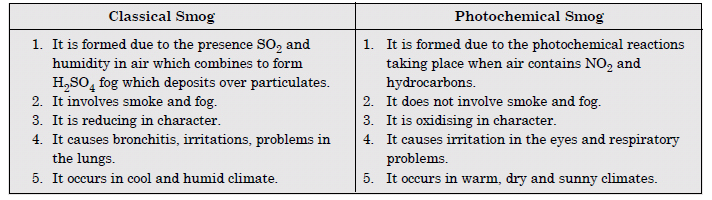
Question 7:
Write chemical reactions involved during the formation of photochemical smog.
Answer:
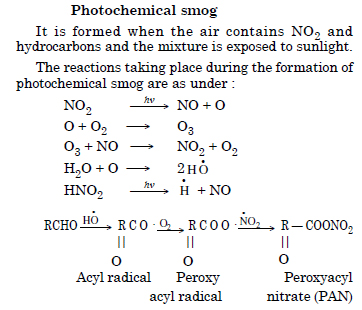
Question 8:
What are the harmful effects of the photochemical smog ? How can they be controlled ?
Answer:
Harmful effects of Photochemical smog The three main components of photochemical smog are nitrogen oxides, ozone and organic derivatives such as acrolein, formaldehyde, PAN etc.
(i) Ozone has pungent smell and it can cause coughing, wheezing, bronchial constriction and irritation to the respiratory mucous system.
(ii) Rubber is cracked and aged by ozone.
(iii) Ozone can cause damage to the vegetation.
(iv) PAN is also toxic to the plants. Control of photochemical smog
(i) By fitting efficient catalytic converters in the automobiles so that emission of nitrogen oxides and hydrocarbons by the automobiles into the atmosphere is prevented.
(ii) By spraying certain compounds into the atmosphere which produce free radicals which readily combine with free radicals which initiates the
formation of compounds which cause photochemical smog.
Question 9:
What are the reactions involved for ozone layer depletion in stratosphere ?
Answer:
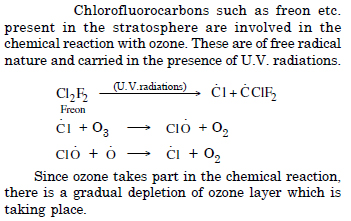
Question 10:
What do you understand by ozone hole ? What are its consequences ?
Answer:
Ozone hole implies destruction of the ozone layer by the harmful ultraviolet (UV) radiations. The depletion will virtually result in creating some sort of holes in the blanket of ozone which surrounds us. As a result, the harmful radiations of sun will cause skin cancer, loss of sight and will also affect our immune system.
Question 11:
What are the major causes of water pollution ? Explain.
Answer:

Question 12:
Have you ever observed any water pollution in your area ? What measures would you suggest to control it ?
Answer:
Yes. The water pollution can be controlled by :
1. Treatment of sewage :
(a) By removing impurities
(b) by passing chlorine
(c) by treatment with alum.
2. Treatment of industrial waste :
(a) By precipitating impurities
(b) By photocatalysis
(c) By using ion exchangers.
Question 13:
What do you understand by Biochemical Oxygen Demand (B.O.D.) ?
Answer:
It may be defined as : the amount of oxygen in milligrams dissolved in water needed by micro-organisms to break down the organic matter present in one litre of water for five days at 20°C. Pure water contains B.O.D. upto 3 ppm. In case, this level is more, it will suggest the presence of organic waste in water which consume oxygen.
Question 14:
Do you observe any soil pollution in your neighbourhood ? What efforts will you make for controlling the solid pollution ?
Answer:
The soil pollution can be controlled by :
(1) By using manures
(2) by using bio-fertilizers
(3) By using proper sewerage system
(4) By salvage and recycling waste products.
Question 15:
What are pesticides and herbicides ? Explain giving examples.
Answer:
Pesticides. These are basically synthetic toxic chemicals with ecological repercursions. Examples : DDT, BHC etc. Herbicides. These are the chemical substances which are used to kill weeds e.g. sod. chlorate, sodium arsenite etc.
Question 16:
What do you understand by green chemistry ? How will it help in decreasing environmental pollution ?
Answer:
Green chemistry is producing the chemicals of daily needs using such reactions and chemical processes which neither use toxic chemicals nor emit such chemicals into the atmosphere. Some achievements of green chemistry are :
(i) Development of new method of synthesizing ibuprofen.
(ii)Designing of a safer marine antifouling compound sea-nine that degrades far rapidly than organotins.
(iii) Development of a method for catalytic dehydrogenation of diethanolamine, resulting in the production of environmentally friendly herbicide.
(iv) Development of processes for the manufacture of polystyrene foam sheet packaging material, using CO 2 as a blowing agent.
Question 17:
What would happen if greenhouse gases were totally missing in earth's atmosphere ? Discuss.
Answer:
Refer to SAQ.
Question 18:
A large number of fish are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you can find an abundance of phytoplankton. Suggest a reason for the fish kill.
Answer:
Phytoplankton growth occurs in water because of the presence of organic matter like leaves, grass, trash etc. in water. It is likely to consume a lot of oxygen dissolved in water which is very much essential for the life of sea animals particularly fish. If the level of dissolved oxygen in water is below 6 ppm, this means that the oxygen is not sufficiently available to the variety of fish living in water. They are likely to perish or die. This may lead to death of fish.
Question 19:
How can domestic waste be used as manure ?
Answer:
Domestic waste consists of both biodegradable and non-biodegradable components. The non-biodegradable waste consisting of plastic, glass, metal scrap etc. is separated from it. The biodegradable waste which consists of organic matter can be converted into manures by using suitable methods.
Question 20:
For your Agricultural field or garden you have developed a compost producing pit. Discuss the process in the light of bad odour; flies and recycling of wastes for good produce.
Answer:
The compost producing pit should be set up at suitable place or in a tin. It should be kept covered. The recycleable materials should be recycled by proper industries.
