Question 1:
Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer:
H1 has electronic configuration 1s1 under group I elements hydrogen has one electron in the s-orbital of its valence shell, therefore it is placed along with group elements at their top.
Question 2:
Write the names of the three isotopes of hydrogen. What is the mass ratio of these isotopes ?
Answer:

Question 3:
Why does hydrogen occur in diatomic form rather than in monoatomic form under normal conditions ?
Answer:
In monoatomic form hydrogen atom has only one electron in K-shell (1s 1) while in diatomic form, the K-shell is complete (1s2). This means that in diatomic form, hydrogen (H2) has acquired the configuration of nearest noble gas, helium. It is therefore, quite stable.
Question 4:
How can the production of dihydrogen from ‘coal gasification’ be increased ?
Answer:
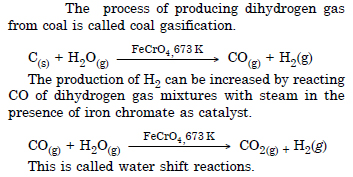
Question 5:
Describe the bulk preparation of dihydrogen by electrolytic method. What is the role of an electrolyte in the process ?
Answer:
Dihydrogen is formed mainly from water by carrying its electrolysis in the presence of suitable
electrolytes i.e., small amount of an acid or alkali using Pt electrodes. Water as such is a poor conductor of
electricity. Addition of small amount of electrolyte increases its conductivity.
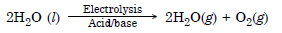
Question 6:
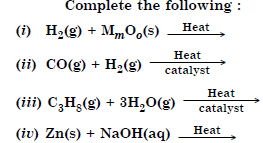
Answer:
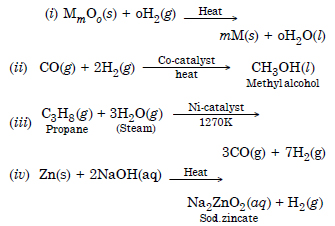
Question 7:
Describe the consequences of high bond enthalpy of H–H bond in terms of chemical reactivity of dihydrogen.
Answer:

Question 8:
Why do you understand by
(i) electron deficient
(ii) electron precise and
(iii) electron rich compounds of hydrogen ? Provide
justification with suitable examples.
Answer:
(i) Electron deficient compounds of hydrogen. They have lesser number of electrons than
required for conventional Lewis structure e.g. B2H6.
(ii) Electron precise compounds of hydrogen. They have the required number of electrons for conventional Lewis structures e.g. CH3.
(iii) Electron rich compounds of hydrogen. They have excess electrons which are present as lone pairs e.g. Ml2, H2O.
Question 9:
What characteristics do you expect from electron deficient hydride with respect to its structure and chemical reaction ?
Answer:
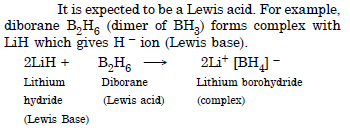
Question 10:
Do you expect the carbon hydride of the type (CnH2n+2) to act as Lewis acid or base ? Justify your answer.
Answer:
It is neither a Lewis acid or nor a Lewis base. In all the hydrides belonging to this type, carbon atoms have complete octet. Therefore, these hydrides behave as normal covalence hydrides.
Question 11:
What do you understand by the term non-stoichiometric hydrides ? Do you expect the types of hydrides to be formed from alkali metals ? Justify your answer.
Answer:
These are formed by many d- & f- block elements and hydrogen occupy interstices in the molal metal lattice
e.g. T. H1.5 – 1.6, Zn H1.3 – 1.2s Alkali metals do not form these type of hydrides because in their crystal
lattices atoms of hydrogen which are very small in size of not fit in.
Question 12:
How would you expect the metallic hydrides to be useful for hydrogen storage ? Explain.
Answer:
Some metals like palladium (Pd), platinum (Pt) have a tendency to adsorb large volume of hydrogen on their surface resulting in forming hydrides. Thus hydrogen gets stored and can be released whenever required.
Question 13:
How does atomic hydrogen or oxyhydrogen torch function for cutting and welding purposes ? Explain.
Answer:
Atomic hydrogen torch.When molecular hydrogen is passed through tungsten electric arc ~ 3000°C, at low pressure, it
dissociates to form atoms of hydrogen known as atomic hydrogen
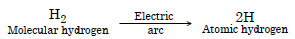
Question 14:
Among NH3, H2O and HF which would you expect to have highest magnitude of hydrogen bonding and why ?
Answer:
The strength of H-bonding depends upon the magnitude of the polarity of the bond. Since H–F bond is maximum polar (F is the most electronegative
element) hydrogen bonding is the maximum in HF
molecules.
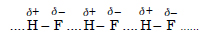
Question 15:
Saline hydrides are known to react violently water producing fire. Can CO2, a well known extinguisher, be used in this case ? Explain.
Answer:
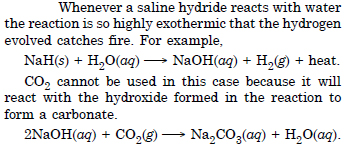
Question 16:

Answer:

Question 17:
Compare the structure of H2O and H2O2.
Answer:
Refer to SAQ; Q. No. 58.
Question 18:
What do you understand by ‘autoprotolysis’ or water ? What is its significance ?
Answer:
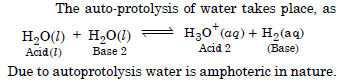
Question 19:
Consider the reaction of water with F2 and suggest in terms of oxidation and reduction which species are oxidised/reduced.
Answer:
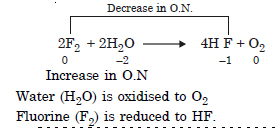
Question 20:
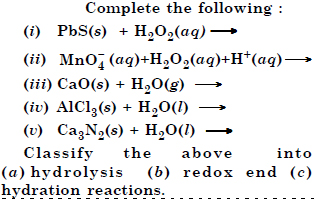
Answer:
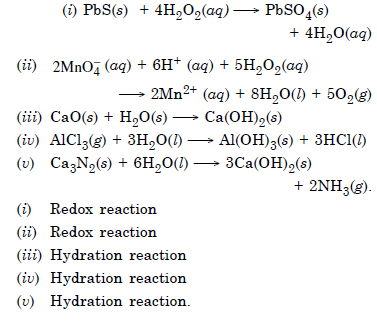
Question 21:
Describe the structure of common form of ice.
Answer:
Refer to Additional Important Qs. (SAQ); Q. No. 64.
Question 22:
What causes temporary and permanent hardness of water ?
Answer:
Refer to Additional Important Qs (SAQ); Q. No. 65 (ii).
Question 23:
Discuss the principle and method of softening of hard water by synthetic ionexchange method.
Answer:
Refer to Additional Important Qs. (LAQ); Q. No. 4.
Question 24:
Write chemical reactions to show amphoteric nature of water.
Answer:
Refer to Additional Important SAQ; Q. No. 66.
Question 25:
Hydrogen peroxide can act both as an oxidising as well as reducing agent. Explain.
Answer:
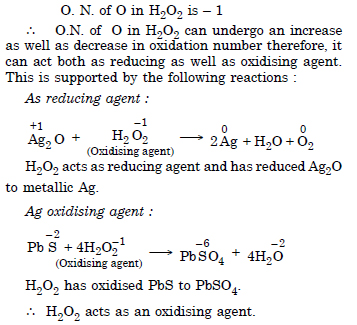
Question 26:
What is meant by demineralised water ? How can it be obtained ?
Answer:

Question 27:
Is dimineralised or distilled water always useful for drinking purposes ? If not, how it can be made useful ?
Answer:
No, dimineralised water is not always useful for drinking purposes. It is usually tasteless. Moreover, some ions such as Na+, K+ and Mg2+ etc. are essential to the body. In order to make dimineralised water more useful, additional salts of sodium and potassium etc. must be dissolved in it.
Question 28:
Describe the usefulness of water in biosphere and biological systems.
Answer:
A major part of all living organisms is made of water. It is a crucial compound for the survival of all forms of life. It is a solvent of great importance.
Question 29:
What properties of water make it useful as a solvent ? What type of compounds can it dissolve and (ii) hydrolyse ?
Answer:
Water is a useful, rather excellent solvent due to the following properties :
(a) It is a liquid over a wide range of temperature (0° to 100°C).
(b) It has high enthalpy of vaporisation and heat capacity.
(c) It is polar in nature and high dielectric constant (78.39). It can dissolve polar substances and also some
organic compounds due to hydrogen bonding. Water can hydrolyse oxides, halides, phosphides, nitrides etc.
Question 30:
Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes.
Answer:
No, heavy water (D2O) is not useful for drinking purposes. Rather it is injurious to health due to the presence of D+ ions.
Question 31:
What is the difference between hydrolysis and hydration ?
Answer:

Question 32:
How can saline hydrides remove traces of water from organic compounds ?
Answer:
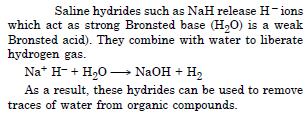
Question 33:
What do you expect the nature of hydrides if formed by the elements of atomic numbers 15, 19, 23 and 44 with dihydrogen ?
Answer:
The elements with atomic numbers 15, 19, 23 and 44 are phosphorus (P), potassium (K), titanium (Ti) and ruthenium (Rh). The nature of their respective hydrides is : PH3 (Molecular), K+H– (Ionic) interstitial or metallic hydrides with titanium (Ti) and ruthenium (Rh).
Question 34:
Do you expect different products in solution when aluminium (III) chloride and potassium chloride treated separately with
(i) normal water
(iii) acidified water and
(iii) alkaline water.
Answer:
Both the compounds are salts and they react differently with water. Aluminium (III) chloride or AlCl3 will react with water as follows :
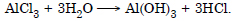
(i) In normal water, both (Al(OH)3 and HCl will be present.
(ii) In alkaline water, HCl will be neutralised by the alkali added. Therefore, it will contain mainly Al(OH)3 and it will be alkaline in nature.
(iii) In acidic water. In this medium, Al(OH)3 will be neutralised by the acid added. Therefore, water will contain mainly HCl and will be acidic in nature. Potassium chloride is a salt of strong acid and strong base. It will remain as such under all the conditions and will not undergo any chemical reaction
Question 35:
How does H2O2 behave as a bleaching agent ?
Answer:
Question 36:
What do you understand by the terms:
(i) hydrogen economy
(ii) hydrogenation (iii) syn gas
(iv) water-gas shift reaction
(v) fuel cell.
Answer:
For (i), (ii), (iii), (iv) – Refer to SAQ. (v) Fuel Cell : It is a commercial cell in which the energy produced during the combustion of fuel appears is the form of electricity, e.g. H2 – O2 fuel cell.
