Question 1:
Explain the formation of a chemical bond.
Answer:
Basics & Basis
Question 2:
Write Lewis dot symbols for atoms of the following elements : Mg, Na, B, O, N, Br.
Answer:
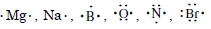
Question 3:
Write Lewis dot symbols for the following atoms and ions : S and S2– ; Al and Al3+; H and H–.
Answer:
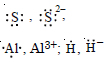
Question 4:
Draw Lewis structures for the following molecules and ions : H2S, SiCl4, BeF2, CO3 2–, HCOOH.
Answer:
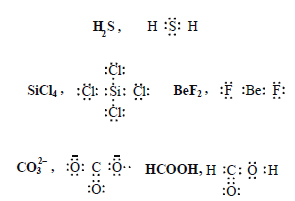
Question 5:
Define Octet Rule. Write its significance and limitations.
Answer:
Octet Rule. According to this rule, atoms can combine either by transfer of valence electrons
from one atom to another or by sharing of valence electrons in order to have an octet in their valence shells.
Significance. It can explain the formation of most of the molecules. Limitations.
(1) In some compounds, the number of electrons surrounding the central atom is less than eight, e.g., BeH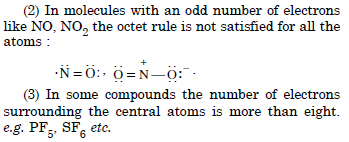
Question 6:
Write the favourable factors for the formation of ionic bond.
Answer:
Refer to ‘Basics & Basis’.
Question 7:
Discuss the shapes of the following molecules using VSEPR model : BeCl2, BCl3, SiCl4, AsF5, H2S, PH3.
Answer:
Refer to Additional Important Questions (LAQs)
Question 8:
Although geometries of NH3 and H2O molecules are distorted, bond angle in water is less than that of Ammonia. Discuss.
Answer:
Refer to Q. No. 33,Additional Important Questions (SAQs)
Question 9:
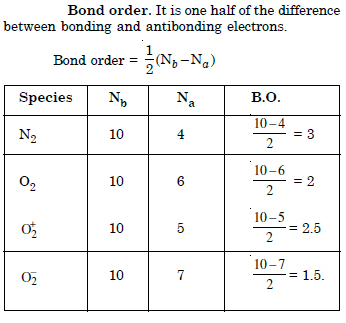
How do you express the bond strength in terms of bond order ?
Answer:
Bond strength is inversely proportional to bond order. The smaller the bond order the stronger their bond will be :
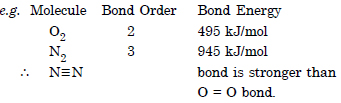
Question 10:
Define the bond length.
Answer:
(a) Bond length. It is the distance of separation between the nuclei of two bonded atoms in a molecule.
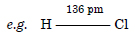
Question 11:
Explain the important aspects of resonance with reference to the CO3 2– ion.
Answer:
Resonance. When all the properties of a substance could not be explained by its single molecular structure and a number of structures have to be
assigned, the actual structure is a resultant of all these contributing structures, known as resonance hybrid and the phenomenon is known as resonance.
Carbonate ion CO3 2– is a resonance hybrid of the following structures :
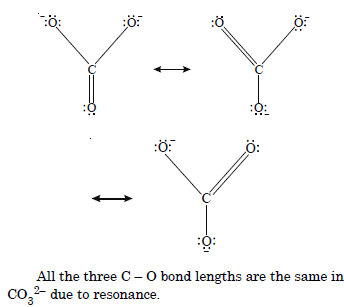
Question 12:
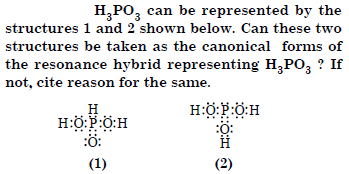
Answer:
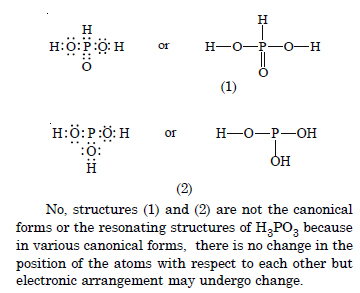
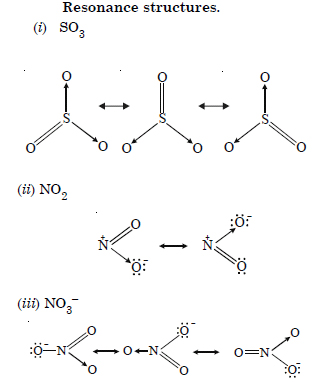
Question 13:
Answer:
Question 14:
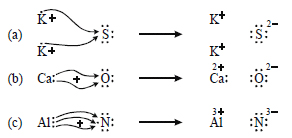
Use Lewis dot symbols to show electron transfer between the following atoms to form cations and anions :
(a) K and S,
(b) Ca and O,
(c) Al and N.
Answer:
Question 15:
Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent (or V-shaped) while that of CO2 is linear. Explain this on the basis of dipole moment.
Answer:
Refer to Additional Important Questions (SAQs) Q.No. 41
Question 16:
Write the significance/applications of dipole moment.
Answer:
Refer to Additional Important Questions (SAQs) Q.No. 43
Question 17:
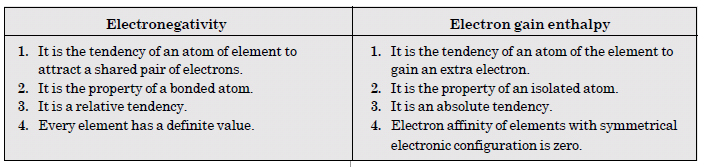
Define electronegativity. How does it differ from electron gain enthalpy ?
Answer:
Electronegativity of an element is the relative tendency of its atom to attract the shared pair of electrons towards itself in a covalent bond. Electronegativity has no units.
Question 18:
Explain with the help of suitable example polar covalent bond.
Answer:
Polar covalent bond A covalent bond formed between two atoms having different electronegativities is called polar covalent
bond. Examples.
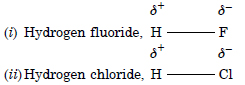
Question 19:
Arrange the bonds in order of increasing ionic character in the molecules : LiF, N2, K2O, SO2 and ClF3.
Answer:
Increasing ionic character is as follows : N2 < SO2 < ClF3 < LiF < K2O.
Question 20:
The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are wrongly shown. Write the correct Lewis
structure for acetic acid.
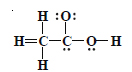
Answer:
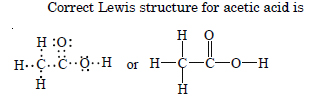
Question 21:
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square-planar ?
Answer:
In CH4 molecule, four sp3 hybridised orbitals are to arrange themselves in space for minimum repulsion and maximum stability which is attained in a tetrahedral arrangement (bond angle = 109° 28') and not in square planar arrangement (bond angle = 90°).
Question 22:
Explain, why BeH2 molecule has zero dipole moment although the Be-H bonds are polar.
Answer:
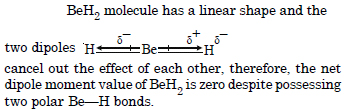
Question 23:
Which out of NH3 and NF3 has higher dipole moment and why ?
Answer:
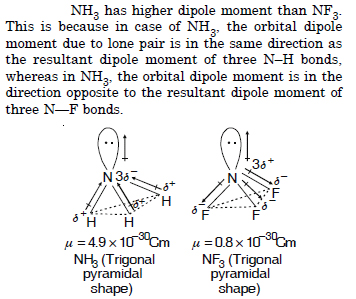
Question 24:
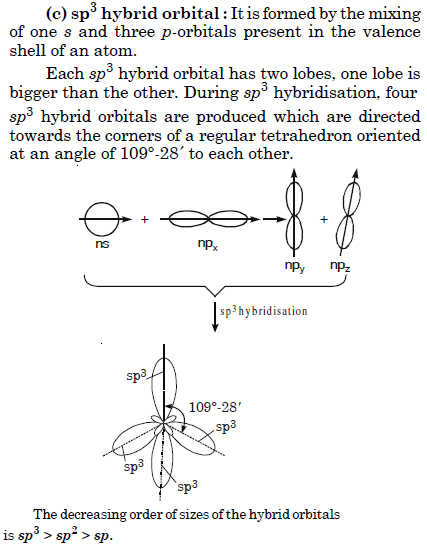
What is meant by hybridisation of atomic orbitals ? Describe the shapes of sp, sp2 and sp3 hybrid orbitals.
Answer:
Hybridisation. The process of mixing up of two or more atomic orbitals of an atom having same
or slightly different energies resulting in the redistribution of their energies so as to form new orbitals (or hybrid orbitals) having equivalent energies
and identical shapes is called hybridisation. Shapes of hybrid orbitals
(a) sp hybrid orbital.It is formed by the mixing up of one s and p orbital present in the valence shell of an atom. It has two lobes,
one lobe is bigger than the other.
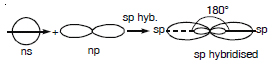
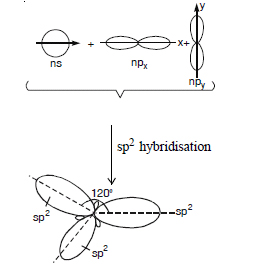
(b) sp2 hybrid orbital. It is formed by the mixing up of one s and two p orbitals present in the valence shell of an atom. Each
sp2 hybrid orbital has two lobes, one lobe is bigger than the other. There are three sp2 hybrid orbitals produced
by the mixing up of one ns and two ns orbitals and these lie in the same plane oriented at an angle of 120°
to each other.
Question 25:
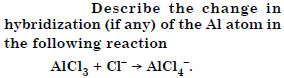
Answer:
Hybridization of atomic orbitals is the concept of intermixing of the atomic orbitals of comparable energy in such a way that the
redistribution of energy takes place and a set of new orbitals is formed, in which orbitals have equivalent energy and identical shapes.

Question 25:
Is there any change in the hybridization of the B and N atoms as a result of the following reaction :
Answer:
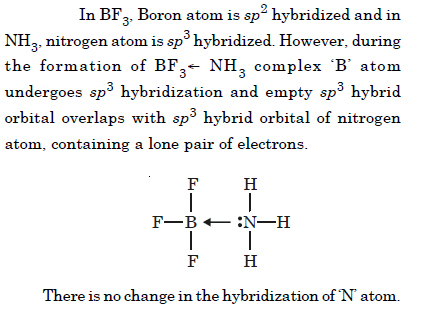
Question 25:
Draw diagrams showing the formation of a double and triple bond between carbon atoms in the C2H4 and C2H 2molecules.
Answer:
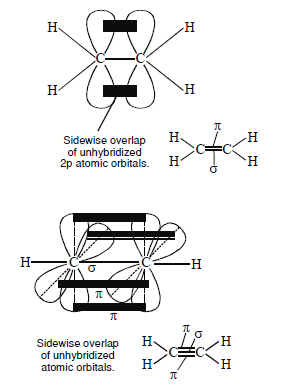
Question 26:
What is the total number of sigma and pi bonds in the following molecules ? (a) C2H2 (b) C2H4.
Answer:
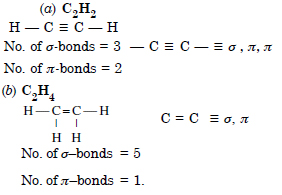
Question 27:
Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why ?
Answer:
2py and 2py
Question 28:
Which hybrid orbitals are used by carbon atoms in the following molecules :
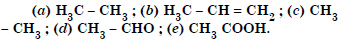
Answer:
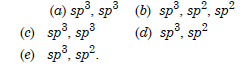
Question 29:
What do you understand by bond pairs and lone pairs of electrons ? Illustrate by giving one example of each type.
Answer:
Bond Pair of electrons. An electron pair which is shared between two combining atoms is called a bond pair.
Lone Pair of electrons. An electron pair which is present in the valence shell of an atom and is not shared with other atoms is called lone pair of electrons.
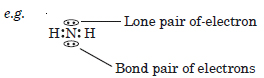
Question 30:
Distinguish between a sigma and a pi-bond.
Answer:
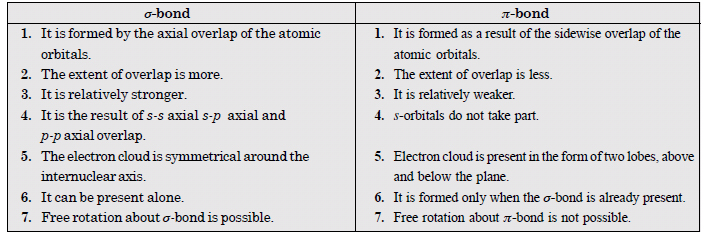
Question 31:
Explain the formation of H2 molecule on the basis of valence bond theory.
Answer:
Refer to Q. No. 1, Additional Imp. Questions (LAQs).
Question 32:
Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals ?
Answer:
Conditions for the linear combination of atomic orbitals to form molecular orbitals :
(1) The combining atomic orbitals should have same or nearly same energies.
(2) The combining atomic orbitals should have same symmetry about the internuclear axis.
(3) The combining atomic orbitals should overlap to a large extent.
Question 33:
Use molecular orbital theory to explain why the Be2 molecule does not exist.
Answer:
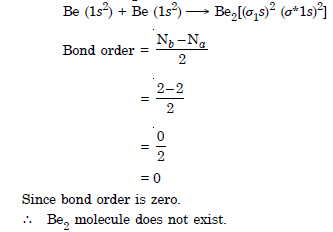
Question 34:
Answer:
Refer to Q. No. 14 Additional Important Questions (LAQs).
Question 35:
Write the significance of a plus and a minus sign shown in representing the orbitals.
Answer:
The plus and a minus sign shown in representing the orbitals indicate the sign of wavefunction and have no link with charge.
Question 36:
Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds ?
Answer:
(Refer to Q. No. 16 Additional Important Questions (LAQs)
Question 37:
(a) Define hydrogen bond.
(b) What are the conditions for H– bond formation ?
(c) What are the types of hydrogen bonds ? Give one example in each case.
(d) Is it (H–bond) weaker or stronger than van der Waals’ forces ?
Answer:
(a) Hydrogen bond. The weak electrostatic forces of attraction between the covalently bonded H–atoms of one molecule and highly electronegative atom (N, O, F) of the same or different molecule is called hydrogen bond.
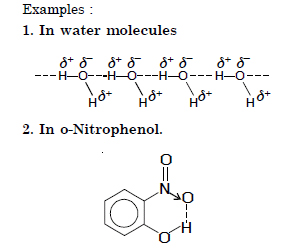
1. Small size. H–atom should be covalently bonded to an atom having small size.
2. High Electronegativity. H–atom should be covalently bonded to an atom having high electronegativity such as F, N and O.
(c) Types of Hydrogen bonds. These are of two types :
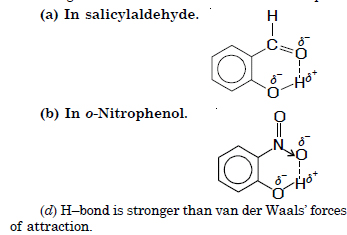
1. Intermolecular H–bond. It is the weak electrostatic force of attraction between covalently bonded H–atom of one molecule and highly
electronegative atom (F, N, O) of the different molecule.
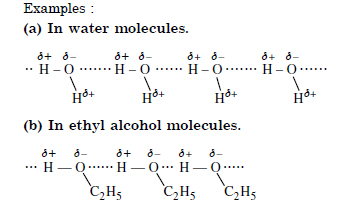
Question 38:
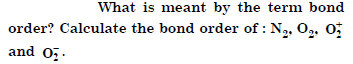
Answer: