Question 1:
Choose the correct answer.
A thermodynamic state function is a quantity :
Answer:
whose value is independent of path
Question 2:
For the process to occur under adiabatic conditions, the correct condition is :
Answer:
dq = 0
Question 3:
The enthalpies of all elements in their standard states are :
Answer:
zero
Question 4:

Answer:
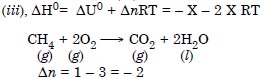
Question 5:

Answer:
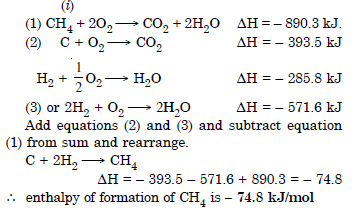
Question 6:
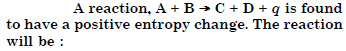
(ii) possible only at low temperature
(iii)not possible at any temperature
(iv) possible at any temperature.
Answer:
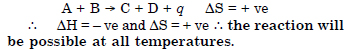
Question 7:
In a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy for the process ?
Answer:

Question 8:
The reaction of cyanamide, NH2CN (s), with dioxygen was carried out in a bomb calorimeter, and △U was
found to be – 742.7 kJ mol–1 at 298 K. Calculate enthalpy change for the reaction at 298 K.
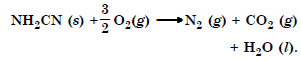
Answer:
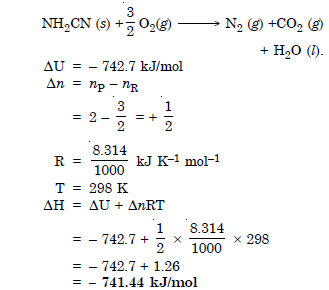
Question 9:
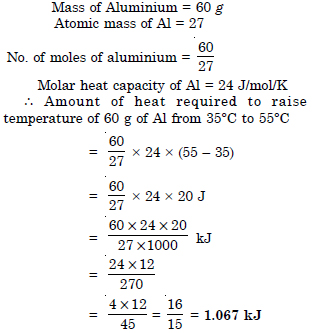
Calculate the number of kJ necessary to raise the temperature of 60.0 g of aluminium from 35 to 55° C. Molar heat capacity of Al is 24 J mol–1 K–1.
Answer:
Question 10:
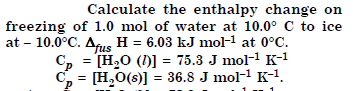
Answer:
(a) Bond length. It is the distance of separation between the nuclei of two bonded atoms in a molecule.
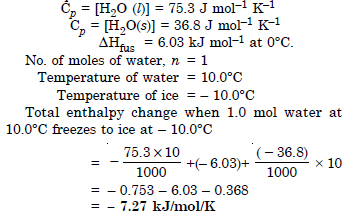
Question 11:
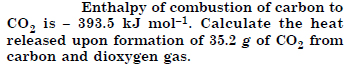
Answer:
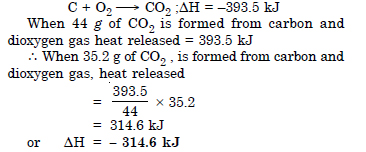
Question 12:
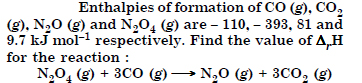
Answer:
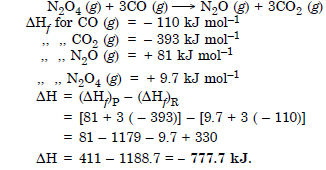
Question 13:
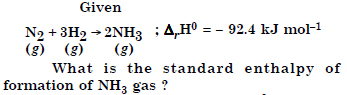
Answer:
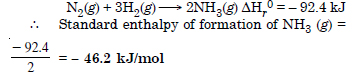
Question 14:
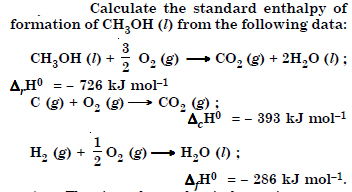
Answer:
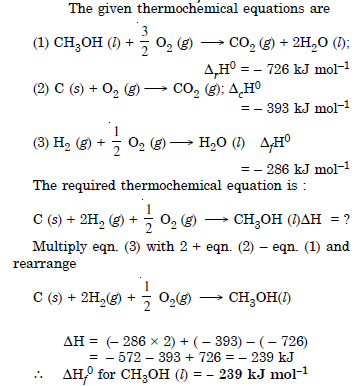
Question 15:
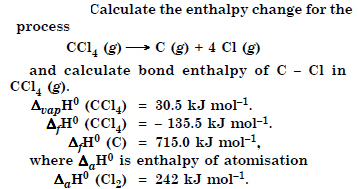
Answer:
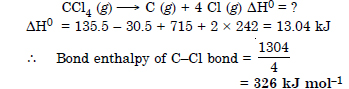
Question 16:
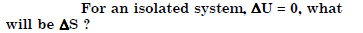
Answer:
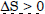
Question 17:

Answer:

Question 18:
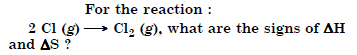
Answer:
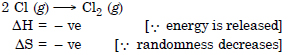
Question 19:

Answer:
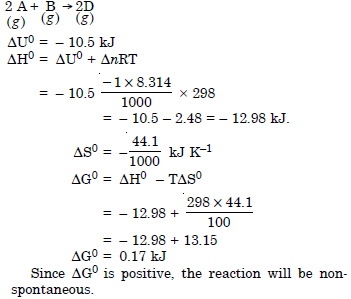
Question 20:
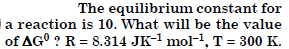
Answer:
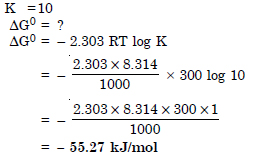
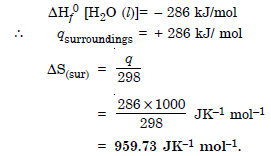
Question 21:
Comment on the thermodynamic stability of NO (g), given
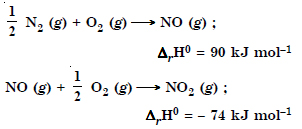
Answer:
NO (g) is unstable, but NO2 is stable and is formed.
Question 22:
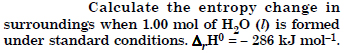
Answer: