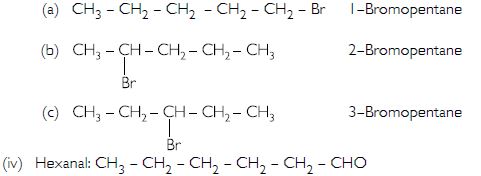Question 1:
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer:
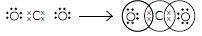
Question 2:
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint: The eight atoms are joined together in the form of a ring.)
Answer:
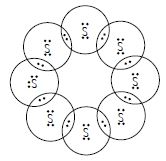
Question 3:
How many structural isomers can you draw for pentane?
Answer:
Pentane has three structural isomers. These are:
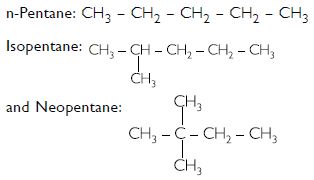
Question 4:
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer:
These are 1. Tetracovalency 2. Catenation.
Question 5:
What will be the formula and electron dot structure of cyclopentane?
Answer:
Formula of cyclopentane is C5H10 and its electron dot structure is
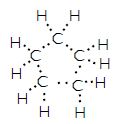
Question 6:
Draw the structures of the following compounds:
- Ethanoic acid
- Bromopentane
- Butanone
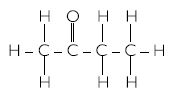
- Hexanal
Are structural isomers possible for bromopentane?
Answer:
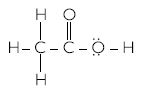
-
Ethanoic acid has the structure:
-
Butanone:
-
Bromopentane: There are three structural isomers possible for bromopentane:
Question 7:
How would you name the following compounds?
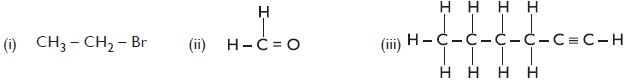
Answer:
- 1-Bromoethane
- Methanal
- Hex-1-yne.
Question 8:
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer:
This is because in this reaction oxygen gets added to ethanol.
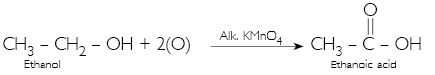
Question 9:
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer:
A mixture of ethyne and air is not burnt for welding because air also contains nitrogen along with oxygen. Nitrogen burn in oxygen producing oxides of nitrogen such as nitric oxide (NO) and nitrogen dioxide (NO2) which cause pollution.
Question 10:
How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer:
The following two tests are used:
- Litmus test. Treat the given compound with blue litmus solution. If the blue litmus solution turns red, it is a carboxylic acid and if does not turn red, it is an alcohol.
- Sodium bicarbonate test. Add some sodium bicarbonate solution to the given compound. If there is a brisk effervescence of a colourless and odourless gas (CO2) which turns freshly prepared lime water milky, it is carboxylic acid. If there is no effervescence, it is an alcohol.
Question 11:
What are oxidising agents?
Answer:
The substances which can oxidise other substances by loosing oxygen are called oxidising agents.
Examples are alkaline potassium permanganate solution, acidified potassium dichromate solution etc.
Question 12:
Would you be able to check if water is hard using a detergent?
Answer:
No, we can’t check whether the water is hard or soft using a detergent. This is because detergent can produce lather even with hard water.
Question 13:
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Answer:
This is because when soap molecules dissolve in dirt, the dirt is somewhat loosened from the clothes and in order to remove it from clothes, the clothes have to be agitated. For that clothes are beaten on a stone or beaten with a paddle or scrubbed with a brush or the mixture is agitated in washing machines.
Question 14:
Ethane, with the molecular formula C2H6, has
- 6 covalent bonds
- 7 covalent bonds
- 8 covalent bonds
- 9 covalent bonds
Answer:
7 covalent bonds
Question 15:
Butanone is a four-carbon compound with the functional group:
- Carboxylic acid
- Aldehyde
- Ketone
- alcohol
Answer:
Ketone
Question 16:
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
- The food is not cooked completely
- The fuel is not burning completely
- The fuel is wet
- The fuel is burning completely
Answer:
The fuel is not burning completely.
Question 17:
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
The formation of CH3Cl can be represented as:
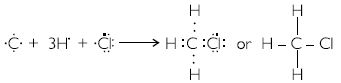
Carbon forms single convalent bonds with three H – atoms and one Cl – atom by sharing one electron pair with each C – H bonds are non-polar. But C – Cl bond is polar because C and H leave almost same electronegativity whereas Cl has more electronegativity than carbon.
Question 18:
Draw the electron dot structures for :
- Ethanoic acid
- H2S
- Propanone
- F2
Answer:
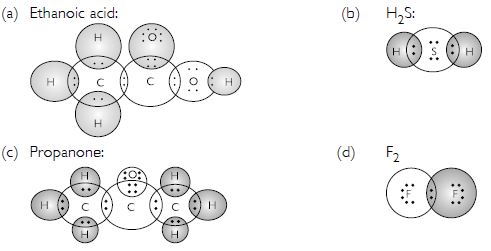
Question 19:
What is a homologous series? Explain with an example.
Answer:
A series of compounds having similar structural formulae, same functional group and hence similar chemical properties is called a homologous series. In the homologous series any two adjacent members differ by a CH2 unit in their molecular formulae.
For example, homologous series of aldehydes (or alkanals) can be represented as:
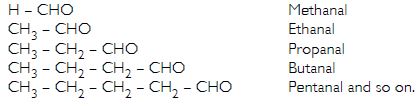
Question 20:
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer:
Differences between ethanol and ethanoic acid:
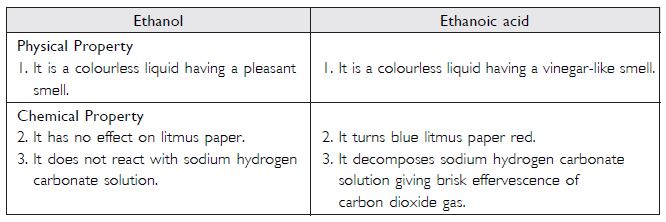
Question 21:
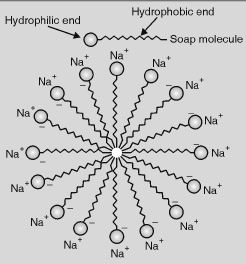
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer:
A soap molecule has two ends with different properties. One end is polar i.e. hydrophilic while the other end is non-polar i.e. hydrophobic. The hydrophilic part is water soluble while the hydrophobic part is insoluble in water. When soap is added to water, the hydrophilic end of soap molecules dissolves in water while the hydrophobic end of soap molecules get attracted to each other, which results in the formation of spherical ionic micelles.The hydrophilic end of soap molecule is insoluble in ethanol; thus micelle formation does not occur in ethanol.
Question 22:
Why are carbon and its compounds used as fuels for most applications?
Answer:
Carbon and its compounds are used as fuels for most applications because they produce a large amount of heat on burning, have low ignition temperature and cobustion is spontaneous.
Question 23:
Explain the formation of scum when hard water is treated with soap.
Answer:
When soap is added to hard water, it reacts with the soluble calcium and magnesium salts present in the water to give insoluble calcium salt of soap called scum.

Question 24:
What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
Soap solution will turn red litmus paper blue because soap is alkaline in nature.
Question 25:
What is hydrogenation? What is its industrial application?
Answer:
The addition of hydrogen to unsaturated hydrocarbons in the presence of catalysts like palladium, platinum, or nickel to give saturated hydrocarbons is called hydrogenation.
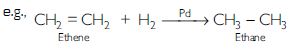
This reaction is used for hydrogenation of liquid vegetable oils using a nickel catalyst to get artificial or vanaspati ghee.
Question 26:
Which of the following hydrocarbons undergo addition reaction: C2H6, C3H8, C3H6, C2H2 and CH4.
Answer:
C3H6 and C2H2 are unsaturated hydrocarbons and undergo addition reactions because they contain multiple bonds. An unsaturated hydrocarbon undergoes addition reactions.
Question 27:
Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer:
Unsaturated compounds are oxidised by alkaline potassium permanganate with the disappearance of its pink colour; therefore when cooking oil (unsaturated compound) is treated with a few drops of alkaline KMnO4 solution, the pink colour of KMnO4 disappears. However with butter, which contains saturated compounds, the pink colour of KMnO4 does not disappear.
Question 28:
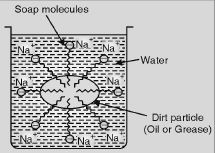
Explain the mechanism of the cleansing action of soaps.
Answer:
Soaps are sodium or potassium salts of higher fatty acids e.g., sodium palmitate, C15H35COO–Na+, sodium sterate, C17H35COO–Na+ etc. A molecule of soap consists of two parts:
- A long chain hydrocarbon part (C15H31 –, C17H35 – etc.) which is soluble in oil and
- Ionic part on polar group, – COO–Na+ which is soluble in water. Thus a molecule of soap can be represented as:
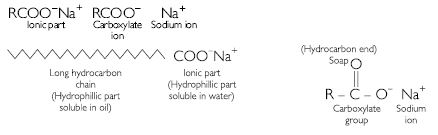
The long hydrocarbon chain is insoluble in water but soluble in oil and greases while the ionic or the polar part is soluble in water. Dirty clothes contain greasy and oily substance (dirt). Soap molecules dissociate in water to give carboxylate ion (RCOO–) and cation (Na+). When dirty clothes are agitated in soap, the hydrocarbon part of carboxylate group dissolves in greasy or oily dirt particles whereas the polar (COO–) group remains attached to water. In this way each oil droplet acquires negative charge. These negative charged oil droplets called micelles cannot coalesce and hence form a stable emulsion in water. These small droplets along with dirt can be easily washed away with water. Thus soap helps in removing greasy dirt by producing stable oil in water-type emulsion. Also, soap reduces surface tension of water. Hence, clothes get cleaned.