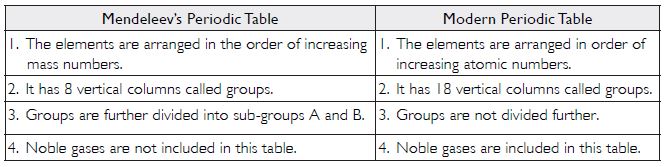
Question 1:
Did Dobereiner’s triads also exist in the columns of Newlands’ octaves? Compare and find out.
Answer:
Yes, Dobereiner’s triads also existed in columns of Newlands’ octaves. These are:
Question 2:
What were the limitations of Dobereiner’s classification?
Answer:
(1) Dobereiner could find only three triads from the elements known at that time.
(2) It was applicable to a few elements.
Question 3:
What were the limitations of Newlands’ law of octaves?
Answer:
(1) It was applicable only up to calcium.
(2) At many places, two elements were put in the same slot in order to adjust elements in
the
Newland’s table.
(3) After the discovery of noble gas, law of octave was not found to be valid.
Question 4:
Use Mendeleev’s periodic table to predict the formulae for the oxides of following elements: K, C, Al, Si, Ba.
Answer:
K2O, CO2, Al2O3, SiO2, BaO
Question 5:
Besides gallium, which other elements have since been discovered to fill the gaps left by Mendeleev in his periodic table? (any two)
Answer:
Scandium and Germanium
Question 6:
What were the criteria used by Mendeleev in creating his periodic table?
Answer:
(1) Mendeleev treated the formulae of hydrides and oxides formed by elements as one of the
basic
properties for their classification.
(2) Mendeleev’s periodic table is based upon Mendeleev’s periodic law which states that the
properties
of the elements are the periodic function of their atomic masses.
Question 7:
Why do you think the noble gases are placed in a separate group?
Answer:
Noble gases were discovered much later than the Mendeleev’s table. Due to their inert nature, these gases were placed in a separate group without disturbing the existing order put forward by Mendeleev.
Question 8:
How could modern periodic table remove various anomalies of Mendeleev’s periodic table?
Answer:
(1) In the modern periodic table, isotopes of an element occupy same position due to the
same
atomic number.
(2) There is a logical separation of elements into groups in modern periodic table.
(3) Modern periodic table is based upon more fundamental property of elements i.e. atomic
number.
Question 9:
Name two elements you would expect to show same kind of chemical reactivity as magnesium. What is the basis for your choice?
Answer:
Calcium and strontium would show chemical reactivity similar to magnesium because they have the same number of valence electrons as magnesium.
Question 10:
Name:
(a) three elements that have a single electron in their outermost shells.
(b) two elements that have two electrons in their outermost shells.
(c) three elements with filled outermost shells.
Answer:
(a) Lithium, sodium and potassium
(b) Magnesium and calcium
(c) Neon, argon and krypton
Question 11:
(a) Lithium, sodium and potassium are all metals that react with water to liberate hydrogen
gas. Is
there any similarity in the atoms of these elements?
(b) Helium is an unreactive gas and neon is a gas of extremely low reactivity. What, if
anything, do
their atoms have in common?
Answer:
(a) The atoms of these elements have one electron in their valence shell.
(b) Atoms of both these elements have compltetely filled valence-shells.
Question 12:
In the modern periodic table, which are the metals among the first ten elements?
Answer:
Lithium and Beryllium
Question 13:
By considering their position in the periodic table, which one of the following elements
would you
expect to have the maximum metallic characteristics?

Answer:
Gallium (Ga)
Question 14:
Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic table?
- The elements become less metallic in nature
- The number of valence electrons increases
- The atoms lose their electrons more easily
- The oxides become more acidic
Answer:
The atoms lose their electrons more easily
Question 15:
Element X forms a chloride with the formula XCl2, which is a solid with a high melting point. X would most likely be in the same group of the periodic table as:
- Na
- Mg
- Al
- Si
Answer:
Mg
Question 16:
Which element has:
(a) two shells, both of which are completely filled with electrons?
(b) the electronic configuration 2, 8, 2?
(c) a total of three shells, with four electrons in its valence shell?
(d) a total of two shells, with three electrons in its valence shell?
(e) twice as many electrons in its second shell as in its first shell?
Answer:
(a) Neon
(b) Magnesium
(c) Silicon
(d) Boron
(e) Carbon
Question 17:
(a) What property do all elements in the same column of the periodic table as boron have in
common?
(b) What property do all elements in the same column of the periodic table as fluorine have
in
common?
Answer:
(a) All elements of this column have 3 electrons in their valence shell.
(b) All elements of this column have 7 electrons in their valence shell.
Question 18:
An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element?
(b) To which of the following elements would it be chemically similar? (atomic numbers are
given in
parenthesis.)
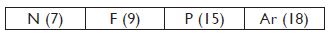
Answer:
(a) 17 (b) F (9) : Electronic configuration (2, 7)
Question 19:
The position of three elements A, B and C in the periodic table are shown below:
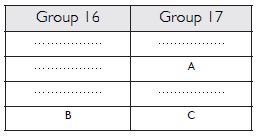
(a) State whether A is a metal or non-metal.
(b) State whether C is more reactive or less reactive than A.
(c) Will C be larger or smaller in size than B.
(d) Which type of ion, cation or anion, will be formed by element A?
Answer:
(a) A is non-metal (b) C is less reactive than A.
(c) C has smaller size than B. (d) Anion, A–
Question 20:
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the periodic table. Write the electronic configurations of these two elements. Which of these will be more electronegative? Why?
Answer:

Nitrogen is more electronegative than phosphorus due to its smaller size.
Question 21:
How does the electronic configuration of an atom relate to its position in the modern periodic table?
Answer:
The electronic configuration of an element tells us about its group number and period number. The number of shells present in an atom of it is equal to its period number and the number of electrons in the valence shell of its atoms is equal to its group number. For example the electronic configuration of element sodium with atomic number 11 is 2, 8, 1. As revealed, a sodium atom has 3 shells thus sodium belongs to 3rd period. Also a sodium atom has one electron in its valence shell. Thus, sodium belongs to first group.
Question 22:
In the modern periodic table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21 and 38. Which of these have physical and chemical properties resembling calcium?
Answer:
Elements with atomic numbers 12 and 38 have physical and chemical properties resembling calcium because they have two electrons in their valence shells like calcium (2, 8, 8, 2).
Question 23:
Compare and contrast the arrangement of elements in Mendeleev’s periodic table and the modern Periodic table.
Answer:
Similarities:
(1) In both, the elements are arranged in groups and periods.
(2) In both, similar elements are placed in same group.
(3) Both classifications make the study of elements simple and systematic.
Dissimilarities: